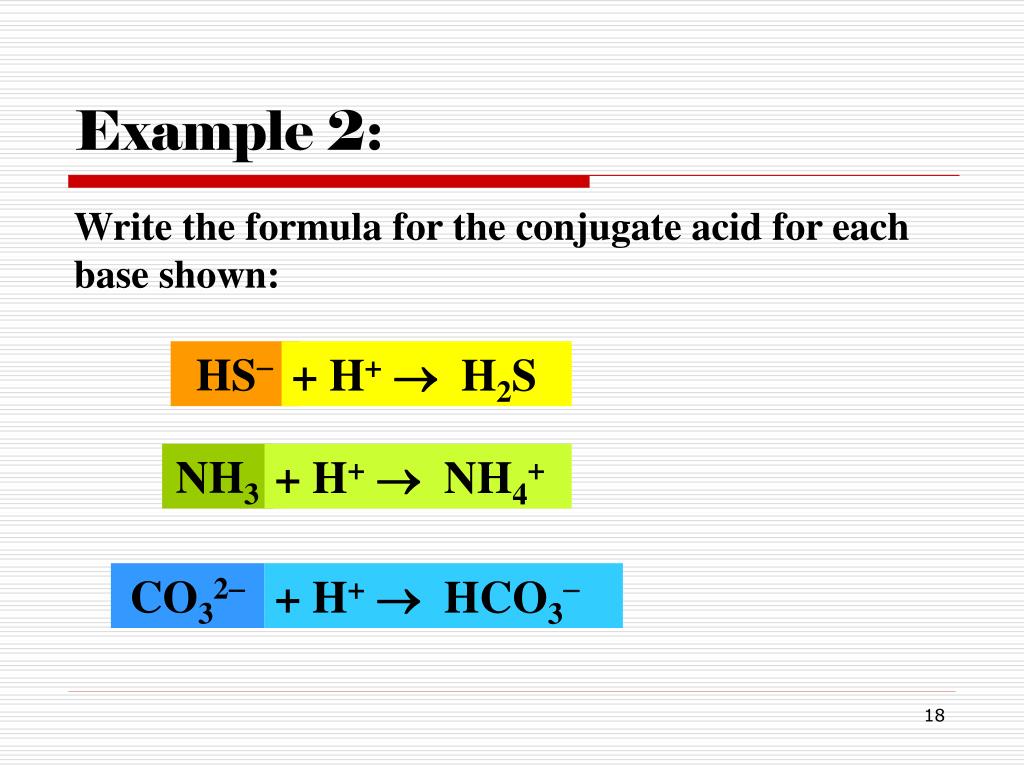
What Is The Conjugate Acid Of Hs - The same idea applies to a base: Conjugate base is the substance formed when an acid loses a hydrogen ion. What is the conjugate base of hcl. Web the conjugated acid of a compound refers to the product that comes out of the reaction when the starting acts as a base. According to this theory an acid is a proton donor while the base is a proton acceptor. Web hydrosulfuric acid is the conjugate acid of the hydrogen sulfite ion it dissociates into in solution. Considered a base because it gains a hydrogen ion to reform the acid. Acids and bases are chemicals that depend on either the donation of hydrogen atoms or the acceptance of electrons. Web it becomes the hydrogen sulfite ion ( h so− 4) which is the conjugate base of sulfuric acid. Web the molecule that accepts a pair of electrons in a reaction is a.
Web the conjugated acid of a compound refers to the product that comes out of the reaction when the starting acts as a base. Acids and bases are chemicals that depend on either the donation of hydrogen atoms or the acceptance of electrons. According to this theory an acid is a proton donor while the base is a proton acceptor. Please answer number 11 and 12: Considered a base because it gains a hydrogen ion to reform the acid. The same idea applies to a base: E.g h3o+ is that of water (h2o). The proton is represented by the. What is the conjugate acid of h s. Web for you to know the conjugate acid simply add an h+. Web the conjugate acid of hs− is. According to brønsted lowry acid base theory,. What is the conjugate base of hcl. Conjugate base is the substance formed when an acid loses a hydrogen ion. 4) given the following acid/base equilibrium assign each species as the bronsted acid, bronsted base, conjugate base, conjugate acid. N h 3 + h 2o <=> n h + 4 + oh − ammonia ( n h 3) is. Web it becomes the hydrogen sulfite ion ( h so− 4) which is the conjugate base of sulfuric acid. H a ( a q) + h 2 o ( l) → a − ( a q) + h 3 o + ( a q) ha is acid because it donates the proton to. Web the molecule that accepts a pair of electrons in a reaction is a. Thus creating a (conjugated) acid.