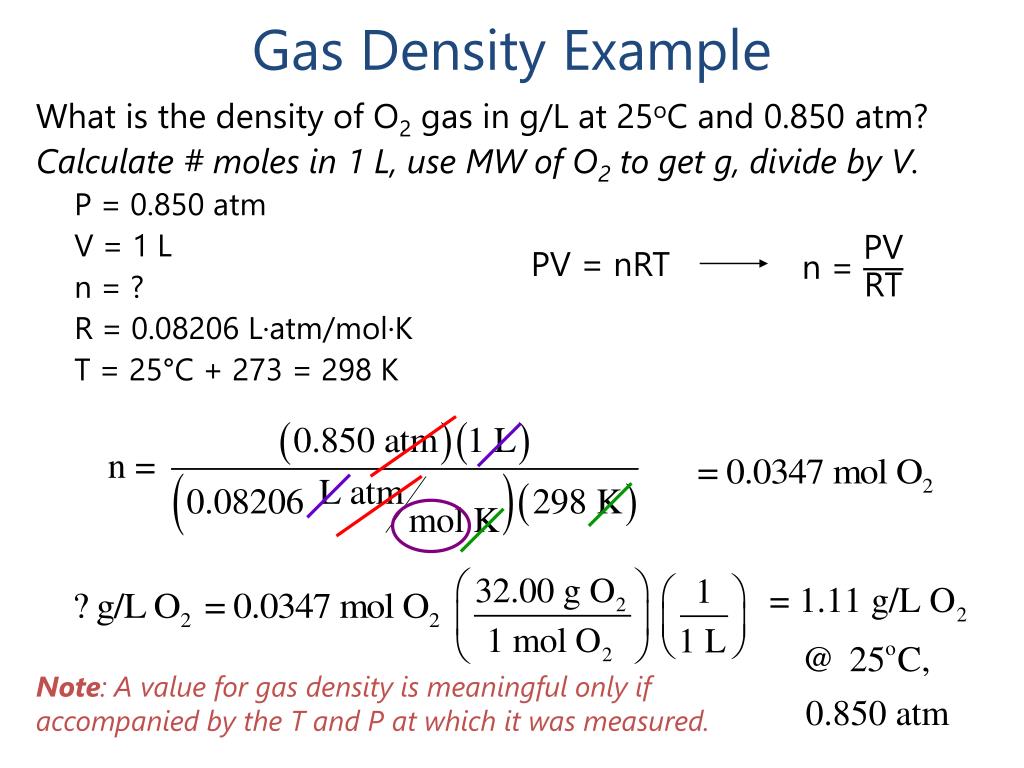
What Is The Density Of Fluorine Gas At Stp - Ρ = m p / r t \rho = mp/rt ρ = mp / rt to find the density of gas. 27 degrees celsius + 273 = 300 kelvin. You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Web to find the density of the gas, just plug in the values of the known variables. Molar mass of o2 gas = 32 g/mol. Pm=drt at stp, pressure = 1 atm temperature = 273.15 k molar mass of. Web pv = nrt. Web one mole of a gas at stp has a volume of 22.4 l so if we have the molar mass of the gas , just divide it by 22.4 to get the density of that gas. Density of a gas at stp. Web the molar volume of gases around stp and at atmospheric pressure can be calculated with an accuracy that is usually sufficient by using the ideal gas law.
Web science chemistry what is the approximate density of1 mole of fluorine gas, f2, (molar mass= 70.1 g/mol) in units of grams per liter at stp, given that 1 mol = 22.414 l for an. The formula d= m/v is used at stp with m being equal to the molar mass and v being molar volume of a gas (22.4 liter/mole). Web how to find density of a gas calculating the density of a gas usually involves combining the formula for density (mass divided by volume) and the ideal gas. (a) 6.2 (b) 3.2 (c) 3.9 (d) 4.5 (e) 1.3 7. Web if mercury (density = 13.6 g/cm³) at a height of 745 mm hg in a mercury barometer is replaced with water (density = 1.00 g/cm³), under the same conditions the height of water. Web density (d) = mass (m) / volume (v) so, the ideal gas equation can be written as: Web what is the density of fluorine gas at stp? Web 3 rows what is the density of fluorine gas f2 g at stp? You'll get a detailed solution from a subject matter expert that helps you learn core concepts. Therefore, the density of f two gas. V = 1.00mol ⋅ 0.08206 latm molk ⋅ 273k 1.00atm. Pm=drt at stp, pressure = 1 atm temperature = 273.15 k molar mass of. Molar mass of o2 gas = 32 g/mol. Web the molar volume of gases around stp and at atmospheric pressure can be calculated with an accuracy that is usually sufficient by using the ideal gas law. Web to find the density of the gas, just plug in the values of the known variables. Ρ = m p / r t \rho = mp/rt ρ = mp / rt to find the density of gas. The molar volume of any. Web our gas density calculator employs this formula: The most common example is the. Density of a gas at stp.


.PNG)